Product Information
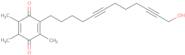
- AA-8612-(12-Hydroxydodeca-5,10-diynyl)-3,5,6-trimethyl-p-benzoquinone
- 2,5-Cyclohexadiene-1,4-dione, 2-(12-hydroxy-5,10-dodecadiyn-1-yl)-3,5,6-trimethyl-
- 2,5-Cyclohexadiene-1,4-dione, 2-(12-hydroxy-5,10-dodecadiynyl)-3,5,6-trimethyl-
- 2-(12-Hydroxy-5,10-dodecadiyn-1-yl)-3,5,6-trimethyl-2,5-cyclohexadiene-1,4-dione
- 2-(12-Hydroxy-5,10-dodecadiynyl)-3,5,6-trimethyl-p-benzoquinone
- 2-(12-Hydroxydodeca-5,10-Diyn-1-Yl)-3,5,6-Trimethylcyclohexa-2,5-Diene-1,4-Dione
- 2-[12-Hydroxydodeca-5,10-diynyl]-3,5,6-trimethyl-p-benzoquinone
- A 61589
- Aa 861
- Docebenona
- See more synonyms
- Docebenona [INN-Spanish]
- Docebenone [USAN:INN]
- Docebenonum
- Docebenonum [INN-Latin]
- Unii-2Xrx3Bd53M
Covalent linkages are the main type of chemical bonds that connect atoms in a molecule together. Docembenone is a coumarin derivative with covalent links which have been shown to inhibit polymerase chain reaction and 5-lipoxygenase. The covalent linkage between the two molecules prevents the enzyme from binding to its substrate, inhibiting the production of leukotrienes and tumor necrosis factor-α. The covalent linkage also inhibits DNA synthesis by blocking DNA polymerases, leading to cellular necrosis and apoptosis.
Docembenone has been shown to cause cancer tissue death by inhibiting DNA synthesis and causing oxidative injury. This drug also binds to antigen-binding molecules on human polymorphonuclear leukocytes, which may be related to its anti-inflammatory effects.
Chemical properties
Technical inquiry about: 3D-FD30173 Docembenone
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.