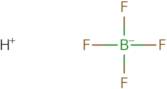
Fluoroboric acid 50% aq
CAS: 16872-11-0
Ref. 3D-FF02671
| 1kg | To inquire | ||
| 50g | To inquire | ||
| 100g | To inquire | ||
| 250g | To inquire | ||
| 500g | To inquire |
Product Information
- Acide fluoborique
- Acide tetrafluoroborique
- Acido Tetrafluoroborico
- Acido fluoborico
- Borate(1-), tetrafluoro-, hydrogen (1:1)
- Borate, Tetrafluoro-, Hydrogen
- Borofluoric acid
- Fluoboric acid
- Fluoboric acid (HBF4)
- Fluoboric acid (HBF<sub>4</sub>)
- See more synonyms
- Fluoborisk syre
- Fluorborsäure
- Fluoroboric acid
- HBF<sub>4</sub>
- Hydrofluoboric acid
- Hydrogen tetrafluoroborate
- Hydrogen tetrafluoroborate(1-)
- Tetrafluorborsäure
- Tetrafluoroborato de hidrógeno
- Tetrafluoroboric Acid
- Tetrafluoroborisk syre
- Tetrafluoroborsaeure
- Tetrafluoroborsaure
- ホウフッ化水素酸
- 四フッ化ホウ酸水素
Fluoroboric acid is a chemical compound with the molecular formula BF3. It is a strong acid that is soluble in water and other polar solvents. The fluorine atom has three lone pairs of electrons, which makes it a reactive species. Fluoroboric acid can be used to remove metal ions from wastewater, such as magnesium and iron, by forming insoluble salts. Fluoroboric acid also reacts with malonic acid to form tetrafluoroborate, which can be used as a reducing agent in organic synthesis. The crystal structures of fluoroboric acid have been determined using x-ray crystallography. Surface methodology experiments have shown that fluoroboric acid adsorbs well to Langmuir monolayers at low concentrations, but not at high concentrations. Trifluoroacetic acid (TFA) is an organic compound that reacts with hydrogen fluoride to produce trifluoroacetic anhydride (TFAA). TFAA can be used for
Chemical properties
Technical inquiry about: 3D-FF02671 Fluoroboric acid 50% aq
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.