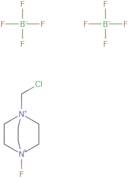
N-Fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate)
CAS: 140681-55-6
Ref. 3D-FF15293
| 5g | 136.00 € | ||
| 10g | 166.00 € | ||
| 25g | 234.00 € | ||
| 50g | 352.00 € | ||
| 100g | 470.00 € |
Product Information
- 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)F-TEDASelectfluorN-Chloromethyl-N?-fluorotriethyle nediammonium bis(tetrafluoroborate)
- 1,4-Diazabicyclo[2.2.2]octane, 1,4-diazoniabicyclo[2.2.2]octane deriv.
- 1,4-Diazoniabicyclo[2.2.2]octane, 1-(chloromethyl)-4-fluoro-, bis[tetrafluoroborate(1-)]
- 1,4-Diazoniabicyclo[2.2.2]octane, 1-(chloromethyl)-4-fluoro-, tetrafluoroborate(1-) (1:2)
- 1-(Chloromethyl)-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane ditetrafluoroborate
- 1-Chloromethyl-4-fluoro-1,4-diazobicyclo[2.2.2]octane bis(tetrafluoroborate)
- 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)
- N-Fluoro-N′-chloromethyltriethylenediamine bis(tetrafluoroborate)
- Selectfluor F-Teda-Bf4
- Selectfluor fluorinating reagent
- See more synonyms
- Selectfluor fluorinating reagent (Selectfluor)
- N-Fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate
- Selectfluor
- Selectfluor
N-Fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate) (FTEDA) is a chemical compound that reacts with hydrogen fluoride to form the triethylammonium bis(tetrafluoroborate) salt. FTEDA is used as a substrate for the fluorination of various organic compounds, such as malonic acid. The reaction mechanism of FTEDA includes an iodination reaction and a reductive elimination reaction. FTEDA has been shown to be effective in treating autoimmune diseases by inhibiting the production of cytokines and antibodies, which are responsible for inflammation.
Chemical properties
Technical inquiry about: 3D-FF15293 N-Fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate)
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.