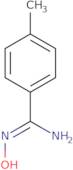
N'-Hydroxy-4-methylbenzenecarboximidamide
CAS: 19227-13-5
Ref. 3D-FH123880
| 1g | Discontinued | ||
| 2g | Discontinued | ||
| 5g | Discontinued | ||
| 10g | Discontinued | ||
| 25g | Discontinued |
Product Information
- benzenecarboximidamide
- N'-hydroxy-4-methyl-
- (2-Methyl-3-Phenylisoxazolidin-5-Yl)Methanol
- 4-Methyl-N-hydroxybenzamidine
- 4-Methylbenzamidoxime
- Art-Chem-Bb B022751
- Benzenecarboximidamide, N-hydroxy-4-methyl-
- Buttpark 33\11-54
- N'-Hydroxy-4-Methylbenzenecarboximidamide
- N-Hydroxy-4-Methyl-Benzamidine
- See more synonyms
- N-Hydroxy-4-Methyl-Benzenecarboximidamid
- N-Hydroxy-4-methylbenzamidine
- N-Hydroxy-4-methylbenzimidamide
- P-Tolamidoxime
- P-Toluamideoxime
- P-Toluamidoxime
- p-Methylbenzamidoxime
- p-Toluamide oxime
- p-Tolylamidoxime
N'-Hydroxy-4-methylbenzenecarboximidamide is a prodrug that is metabolized to the active form, 4-hydroxy-N-methylbenzenecarboximidamide. It has been shown to inhibit prostate cancer cells and may be useful in the treatment of prostate cancer. N'-Hydroxy-4-methylbenzenecarboximidamide inhibits the growth of cancer cells by binding to androstenes, which are natural hormones found in most living organisms. This drug also has an inhibitory effect on the activity of oxadiazole and cyclodehydration, which are enzymes that catalyze reactions involved in cholesterol synthesis. The drug also binds to chlorides, cyanamides, and other halogenated compounds that inhibit DNA synthesis. The mechanism of action for this drug is inhibition of protein synthesis by preventing the formation of peptide bonds between amino acids by blocking the enzyme responsible for this reaction (protein kinase).