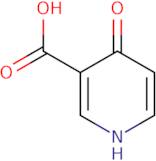
4-Hydroxy-nicotinic acid
CAS: 609-70-1
Ref. 3D-FH12988
| 1g | Discontinued | ||
| 2g | Discontinued | ||
| 5g | Discontinued | ||
| 10g | Discontinued | ||
| 25g | Discontinued |
Product Information
- 3-Pyridinecarboxylic Acid, 1,4-Dihydro-4-Oxo-
- 3-Pyridinecarboxylic Acid, 4-Hydroxy-
- 4-Hydroxy-3-pyridinecarboxylic acid
- 4-Hydroxypyridine-3-carboxylic acid
- 4-Oxo-1,4-dihydropyridine-3-carboxylic acid
- Nicotinic acid, 4-hydroxy-
- 4-Hydroxynicotinic acid
4-Hydroxy-nicotinic acid (4HNA) is a metastable substance that belongs to the group of pyridine derivatives. The redox cycle of 4HNA starts with its reduction to 4-hydroxynicotinamide by intramolecular hydrogen transfer from the amino nitrogen to the hydroxyl oxygen. This reaction requires an activation energy, but does not require any catalysts or cofactors. The second step in the redox cycle is the oxidation of 4-hydroxynicotinamide back to 4HNA by electron donation from an oxidizing agent, such as halides or metal ions. Hydrochloric acid can be used as an oxidizing agent and thermodynamically favors this reaction over the reverse reaction. Kinetically, both reactions are reversible and competitive with each other. Primary cells can be used for cycling experiments in order to study these reactions.