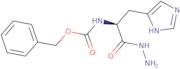
Product Information
- Z-L-histidine hydrazide
- <span class="text-smallcaps">L</span>-Histidine, N-[(phenylmethoxy)carbonyl]-, hydrazide
- Histidine, N-carboxy-, benzyl ester, hydrazide, <span class="text-smallcaps">L</span>-
- N-Benzyloxycarbonyl-<span class="text-smallcaps">L</span>-histidine hydrazide
- N-[(Phenylmethoxy)carbonyl]-<span class="text-smallcaps">L</span>-histidine hydrazide
- N<sup>α</sup>-Benzyloxycarbonyl-<span class="text-smallcaps">L</span>-histidine hydrazide
- benzyl [(2S)-1-hydrazinyl-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]carbamate (non-preferred name)
- benzyl [1-hydrazinyl-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]carbamate (non-preferred name)
- N-Benzyloxycarbonyl-L-histidine hydrazide
- N-[(Phenylmethoxy)carbonyl]-L-histidine hydrazide
- See more synonyms
- Nα-Benzyloxycarbonyl-L-histidine hydrazide
- L-Histidine, N-[(phenylmethoxy)carbonyl]-, hydrazide
- Histidine, N-carboxy-, benzyl ester, hydrazide, L-
Z-His-NH·NH2 is a synthetic substrate that has been used to study the efficiency of catalytic architectures, including serine proteinases and lipases. This substrate has also been used as a prodrug for acetylsalicylic acid (ASA) in order to study its effects on pancreatic catalysis. The structures of Z-His-NH·NH2 have been determined by nuclear magnetic resonance spectroscopy and mass spectrometry.
Chemical properties
Technical inquiry about: 3D-FH47280 Z-His-NH·NH2
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.