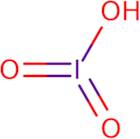
Product Information
- Acide iodique
- Acido Iodico
- Hydrogen iodate
- Hydrogen iodate (HIO3)
- Hydrogen iodate (HIO<sub>3</sub>)
- Iodic Acid
- Iodic acid (HIO<sub>3</sub>)
- Iodsaure
- Jodsaeure
- Iodic acid (HIO3)
- See more synonyms
- Monoiodic acid
- Iodic acid (HIO3)
- magnesium diiodate
- mercury diiodate
- Caswell No. 499A
- EPA Pesticide Chemical Code 046902
Iodic acid is a polycarboxylic acid that is activated by the loss of two protons. Ionic liquids have been shown to have high stability and very low viscosity, which makes them attractive for use as solvents in chemical reactions. Ionic liquids are often used as precursors for ionic polymers, which can be processed into nanoparticles or microparticles. The formation of ionic liquid-based materials depends on the reaction conditions, such as temperature and pressure. This type of material has been used in industrial preparations for the production of trifluoroacetic acid and water vapor. Ionic liquids have also been used in the synthesis of metal salts, such as gold salts.
Ionic liquids are formed when an acylation reaction occurs between an organic acid with a Lewis base, such as ammonia or pyridine. This type of reaction is called Friedel-Crafts acylation and is catalyzed by aluminum chloride or bor
Chemical properties
Technical inquiry about: 3D-FI163218 Iodic acid
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.