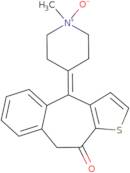
Product Information
- 4,9-Dihydro-4-(1-methyl-1-oxido-4-piperidinylidene)-10H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-one4,9-Dihydro-4-(1-methyl-4-piperid inylidene)-10H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-one N-oxide
Ketotifen N-oxide is the racemic mixture of two enantiomers, (S)- and (R)-ketotifen. It is a chiral compound that has an affinity for transport proteins in the small intestine and lungs. Ketotifen N-oxide is used as a chiral selector in organic synthesis to separate diastereomers. The theory of ketotifen N-oxide's selectivity was first proposed by Emil Fischer, who suggested that it could be due to its ability to form complexes with ionogenic groups on the protein surface. This theory was later confirmed by other chemists who found that the complexation occurs through hydrogen bonding interactions between ketotifen N-oxide and ionic sites on the protein surface.