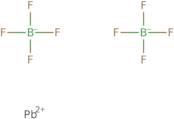
Product Information
- Borate(1-), tetrafluoro-, lead(2+)
- Borate(1-),tetrafluoro-,lead(2+)(2:1)
- Lead Bis(Tetrafluoroborate)
- Lead Fluoborate
- Lead Fluoroborate
- Lead boron fluoride
- Lead fluoborate (Pb(BF<sub>4</sub>)<sub>2</sub>)
- Lead fluoborate,solution(containing>28%)
- Lead fluoroborate (Pb(BF<sub>4</sub>)<sub>2</sub>)
- Lead tetrafluoroborate (Pb(BF<sub>4</sub>)<sub>2</sub>)
- See more synonyms
- Lead(+2)Borofluoride
- Lead(+2)Tetrafluoroborate
- Lead(I)Tetrafluoroboratesolution
- Lead(Ii) Fluoroborate
- Lead(Ii) Tetrafluoroborate
- Leadborofluoride
- Leadboronfluoride
- Leadfluoroborate(Pb(Bf4)2)
- Leadfluoroboratesolution
- Leadtetrafluoroborate
- Leadtetrafluoroborate(Pb(Bf4)2)
- Palladium(2+) Ditetrafluoroborate
- Tetrafluoro-Borate(1-Lead(2+)
- Tetrafluoro-Borate(1-Lead(2+)(2:1)
Lead fluoborate is a chemical compound that has the formula PbF2O4. It is a white solid that is insoluble in water and hydrochloric acid. It can be prepared by the reaction of lead(II) oxide with fluoboric acid. Lead fluoborate has been used as an additive to glass, ceramics, and enamel glazes, as well as a pigment in paints. Lead fluoborate has poor thermal stability and decomposes when heated to temperatures above 450°C (850°F). Lead fluoborate also exhibits low solubility in water, with only 2 g dissolving in 100 mL of water at 25°C (77°F). This property makes it useful for some types of radiation detectors.
The compound undergoes coordination chemistry reactions with metal ions, such as Fe3+, Mn2+, Cu2+, Zn2+, and Co2+. These reactions are not reversible due to the stability of the complex formed