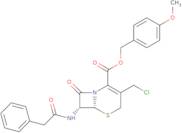
4-Methoxybenzyl 3-chloromethyl-7-(2-phenylacetamido)-3-cephem-4-carboxylate
CAS: 104146-10-3
Ref. 3D-FM25269
| 2g | To inquire | ||
| 5g | To inquire | ||
| 10g | To inquire | ||
| 25g | To inquire | ||
| 50g | To inquire |
Product Information
- (6R,7R)-3-(Chloromethyl)-8-oxo-7-[(2-phenylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (4-methoxyphenyl)meth yl ester(6R-trans)-7-Phenylacetamido-3-(chloromethyl)-3-cephem-4-carboxylic acid p-methoxybenzyl ester
- (4-Methoxyphenyl)methyl (6R,7R)-3-(chloromethyl)-8-oxo-7-[(2-phenylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
- (4-methoxyphenyl)methyl (6R)-3-(chloromethyl)-8-oxo-7-[(2-phenylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
- 4-Methoxybenzyl (6R,7R)-3-(chloromethyl)-8-oxo-7-(2-phenylacetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
- 4-methoxybenzyl (6R,7R)-3-(chloromethyl)-8-oxo-7-[(phenylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
- 5-Thia-1-azabicyclo(4,2,0)oct-2-ene-2-carboxylic acid, 3-(chloromethyl)-8-oxo-7-((phenylacetyl)amino)-, (4-methoxyphenyl)methyl ester, (6R-trans)-
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-(chloromethyl)-8-oxo-7-[(2-phenylacetyl)amino]-, (4-methoxyphenyl)methyl ester, (6R,7R)-
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-(chloromethyl)-8-oxo-7-[(phenylacetyl)amino]-, (4-methoxyphenyl)methyl ester, (6R,7R)-
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-(chloromethyl)-8-oxo-7-[(phenylacetyl)amino]-, (4-methoxyphenyl)methyl ester, (6R-trans)-
- 7-Phenylacetamido-3-(chloromethyl)-3-cephem-4-carboxylic acid p-methoxybenzyl ester
- See more synonyms
- Gcle
Cefozopran hydrochloride is a chemical substance that has been used as an antibiotic preparation. It is a highly soluble, stable substance with low energy and high reactivity. Cefozopran hydrochloride is used to produce cefozopran by reacting with 2-phenylacetamidine and 4-methoxybenzaldehyde in the presence of anhydrous copper chloride and acetic acid at low temperature. The reaction mechanism for this process is the acylation reaction of the amine group with the carboxylic acid group to form an amide bond, which gives rise to a secondary amine. It is then chlorinated by phosphorus pentachloride or trifluoroacetic acid in order to form cefozopran hydrochloride.
Chemical properties
Technical inquiry about: 3D-FM25269 4-Methoxybenzyl 3-chloromethyl-7-(2-phenylacetamido)-3-cephem-4-carboxylate
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.