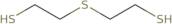2-Mercaptoethyl sulphide
CAS: 3570-55-6
Ref. 3D-FM36235
| 2g | Discontinued | ||
| 5g | Discontinued | ||
| 10g | Discontinued | ||
| 25g | Discontinued | ||
| 50g | Discontinued |
Product Information
- 1,4,7-Trithiaheptane
- 1,5-Dimercapto-3-thiapentane
- 1-Mercapto-2-(mercaptoethylthio)ethane
- 2,2'-Sulfanediyldiethanethiol
- 2,2′-Dimercaptodiethyl sulfide
- 2,2′-Dimercaptodiethyl thioether
- 2,2′-Thiobis[ethanethiol]
- 2,2′-Thiodi(ethanethiol)
- 2-(2-Sulfanylethylsulfanyl)ethanethiol
- 2-Mercaptoethyl sulfide
- See more synonyms
- 2-Mercaptoethyl thioether
- 2-[(2-Mercaptoethyl)thio]ethanethiol
- 2-[(2-Sulfanylethyl)sulfanyl]ethane-1-thiol
- 3-Thia-1,5-pentanedithiol
- Bis(2-mercaptoethyl) sulfide
- Bis(2-sulfanylethyl) sulfide
- Bis(b-Mercaptoethyl) Sulfide,97%
- Ethanethiol, 2,2′-thiobis-
- Ethanethiol, 2,2′-thiodi-
- Iu 11B
- NSC 4766
- TDT
2-Mercaptoethyl sulphide is a synthetic product with the chemical formula CH3SH. It has an absorption band at about 290 nm, which is due to its uv absorption. 2-Mercaptoethyl sulphide has a thiolate group and can be used as an electron donor for coordination chemistry. This compound also has a disulfide bond, which is formed by the oxidation of sulfur. The x-ray crystal structures show that this compound is coordinated to metal ions in such a way that it forms hydrogen bonds with chloride ions. 2-Mercaptoethyl sulphide binds to the receptor site of chloride ion channels, altering their function and inhibiting the flow of chloride ions through them.