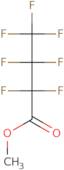
Methyl Heptafluorobutyrate
CAS: 356-24-1
Ref. 3D-FM60486
| 10g | To inquire | ||
| 25g | To inquire | ||
| 50g | To inquire | ||
| 100g | To inquire | ||
| 250g | To inquire |
Product Information
- Heptafluorobutyric Acid Methyl EsterMethyl PerfluorobutyratePerfluorobutyric Acid Methyl Ester
- 2,2,3,3,4,4,4-Heptafluoro-butyric acid methyl ester
- Butanoic acid, 2,2,3,3,4,4,4-heptafluoro-, methyl ester
- Butanoic acid, heptafluoro-, methyl ester
- Butyric acid, heptafluoro-, methyl ester
- Heptafluorobutyryl methyl ester
- Methyl 2,2,3,3,4,4,4-heptafluorobutanoate
- Methyl Heptafluorobutanoate
- Methyl heptafluorobutyrate
- Methyl perfluorobutanoate
- See more synonyms
- Methyl perfluorobutyrate
Methyl heptafluorobutyrate is a reactive compound that is used as a trifluoroacetic acid substitute in analytical chemistry. It can be used to replace toxic or expensive solvents and it has the advantage of being non-flammable, non-explosive, and nontoxic. Methyl heptafluorobutyrate is prepared by the reaction of methylene chloride with hexafluoroacetone at temperatures below -40°C. The resulting product is purified by recrystallization from acetone or benzene. Methyl heptafluorobutyrate is soluble in water and other polar solvents. It has a phase transition temperature of -19°C, which makes it useful for the study of membrane systems at low temperatures. This compound reacts with metal ions such as Fe3+ to form metal chelates, which are stable cations that are useful for chemical analysis. Methyl he
Chemical properties
Technical inquiry about: 3D-FM60486 Methyl Heptafluorobutyrate
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.