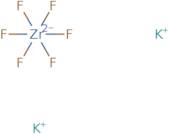
Potassium hexafluorozirconate
CAS: 16923-95-8
Ref. 3D-FP106148
| 1kg | Discontinued | ||
| 2kg | Discontinued | ||
| 500g | Discontinued |
Product Information
- Dipotassium Hexafluorozirconate
- Dipotassium Zirconium(+4) Cation Hexafluoride
- Dipotassium hexafluorozirconate(2-)
- Dipotassium zirconium hexafluoride
- NSC 310011
- Potassium Fluorozirconate
- Potassium Fluozirconate
- Potassium Hexafluorozirconate
- Potassium fluorozirconate (K<sub>2</sub>ZrF<sub>6</sub>)
- Potassium fluozirconate(IV) (K<sub>2</sub>ZrF<sub>6</sub>)
- See more synonyms
- Potassium hexafluorozirconate (K<sub>2</sub>ZrF<sub>6</sub>)
- Potassium hexafluorozirconate(IV)
- Potassium zirconium fluoride (K<sub>2</sub>ZrF<sub>6</sub>)
- Zirconate(2-), hexafluoro-, dipotassium
- Zirconate(2-), hexafluoro-, dipotassium, (OC-6-11)-
- Zirconate(2-), hexafluoro-, potassium (1:2), (OC-6-11)-
- Zirconium Potassium Fluoride
- Zirpro
- Potassium fluozirconate(IV) (K2ZrF6)
Potassium hexafluorozirconate is a diazonium salt that can be used as an analytical reagent to detect the presence of hydroxyl groups. It is activated by sodium carbonate and silver ions, which act as catalysts. Potassium hexafluorozirconate reacts with potassium hexafluorophosphate to form zirconium oxide and hydrochloric acid. This reaction requires heating at a temperature of 80°C for 30 minutes, generating heat in the process. The exothermic reaction produces zirconium oxide and potassium hexafluorophosphate, which are both conditioners for glassware. The cross-linking agent is generated when the two reactants are heated together for an extended period of time.