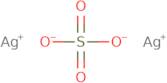
Silver sulfate
CAS: 10294-26-5
Ref. 3D-FS47060
| 5g | Discontinued | ||
| 10g | Discontinued | ||
| 25g | Discontinued | ||
| 50g | Discontinued | ||
| 100g | Discontinued | ||
| 0.1kg | Discontinued | ||
| 0.01kg | Discontinued | ||
| 0.05kg | Discontinued | ||
| 0.005kg | Discontinued | ||
| 0.025kg | Discontinued |
Product Information
- Silver(I) sulfate
- Disilber(1+)sulfat
- Disilver monosulfate
- Disilver sulfate
- Disilver(1+) Sulphate
- Disilver(1+) sulfate
- Silber(I)-Sulfat
- Silver sulfate (Ag2SO4)
- Silver sulfate (Ag<sub>2</sub>SO<sub>4</sub>)
- Sulfate de diargent(1+)
- See more synonyms
- Sulfato De Diplata(1+)
- Sulfuric acid disilver salt
- Sulfuric acid silver salt (1:2)
- Sulfuric acid, silver(1+) salt (1:2)
- Sulfuric acid disilver(I) salt
- silver(1+) hydrogen sulfate
- Sulphuric acid, silver salt
- Sulfuric acid, disilver(1+) salt
- Sulfuric acid, disilver(1+) salt
- sulphuric acid, silver salt
Silver sulfate is the most common silver salt and a potent antimicrobial agent. It is often used to prevent microbial growth in water samples, such as those required for sample preparation or electrochemical impedance spectroscopy. Silver sulfate has been shown to kill bacteria by interfering with their metabolism, leading to cell death. It is also used as an antimicrobial agent in wastewater treatment systems, where it reduces the concentration of hydrogen sulphide. The resistance of cells to silver sulfate is dependent on the redox cycle and the cell's ability to reduce silver ions. Silver sulfates have also been found to cause structural changes in bacterial DNA, which may lead to mutations that would make cells more susceptible to other agents.