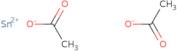
Product Information
- Acetic acid tin(2+) saltTin acetate (sn(OAc)2)
- Acetic acid, tin(2+) salt
- Acetic acid, tin(2+) salt (2:1)
- Stannous acetate
- Tin Di(Acetate)
- Tin acetate
- Tin acetate (Sn(MeCO2)2)
- Tin acetate (Sn(O<sub>2</sub>C<sub>2</sub>H<sub>3</sub>)<sub>2</sub>)
- Tin acetate (Sn(OAc)<sub>2</sub>)
- Tin diacetate
- See more synonyms
- Tin(2+) Diacetate
- TinIIacetateoffwhitepowder
- Tin ii acetate
- Tin acetate (Sn(OAc)2)
Tin(II) acetate is a chemical compound that is stable in air and water, but becomes unstable when heated to decomposition. The metal carbonyl reacts with hydroxyl groups to form the corresponding metal alcoholate. Tin(II) acetate has been shown to be reactive with hydrocarbons, polyols, and other organic compounds. This reaction may be due to the formation of long-chain polymerization products or perovskite crystals. Tin(II) acetate can also undergo a reduction process, leading to the formation of tin metal and hydrogen gas. The resulting product is an insoluble black powder that can be used as a catalyst for various reactions, such as UV irradiation or electrochemical oxidation of organic compounds.