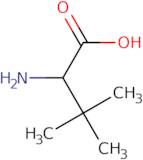
Product Information
- (+/-)-2-Amino-3,3-dimethylbutyric acid
- 2-Amino-3,3-dimethylbutanoic acid
- 3-Methylvaline
- <span class="text-smallcaps">D</smallcap><smallcap>L</span>-tert-Butyl-Glycine
- <span class="text-smallcaps">DL</span>-3,3,3-Trimethyl-2-aminopropionate
- <span class="text-smallcaps">DL</span>-Pseudoleucine
- <span class="text-smallcaps">DL</span>-Terleucine
- <span class="text-smallcaps">DL</span>-Valine, 3-methyl-
- <span class="text-smallcaps">DL</span>-tert-Leucine
- Butyric acid, 2-amino-3,3-dimethyl-, <span class="text-smallcaps">DL</span>-
- See more synonyms
- DL-2-(tert-Butyl)glycine
- DL-tert-Leucine
- H-DL-Tle-OH
- H-tBu-DL-Gly-OH
- Pseudoleucine
- Valine, 3-methyl-
- t-DL-Leucine
- tert-Butylglycine
- tert-Leucine
- ψ-Leucine
- DL-Pseudoleucine
- Butyric acid, 2-amino-3,3-dimethyl-, DL-
- DL-Valine, 3-methyl-
DL-tert-Butylglycine is a chiral compound that is used in the synthesis of enantiopure compounds. This amino acid can be obtained from the reaction of trifluoroacetic acid with l-tert-leucine. It is also used for enzymatic reactions as a substrate and for receptor binding studies. DL-tert-Butylglycine has been shown to bind to bacterial type strains and inhibit their growth by competitive inhibition of the enzyme catalysis. It has been shown to be an enantiomerically pure, racemic mixture of two diastereomers, L-(+)-DL-tert-butylglycine and D-(−)-DL-tert-butylglycine. The conformational properties of this amino acid are dependent on its stereochemistry: it has a chair conformation when it is L(+) and a twist conformation when it is D(−).