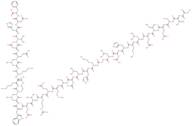
Teriparatide acetate
CAS: 52232-67-4
Ref. 3D-FT41010
| 1mg | Discontinued | ||
| 2mg | Discontinued | ||
| 5mg | Discontinued | ||
| 10mg | Discontinued | ||
| 500µg | Discontinued |
Product Information
- Teriparatide Acetate
- Forteo
- L-Phenylalanine, L-seryl-L-valyl-L-seryl-L-alpha-glutamyl-L-isoleucyl-L-glutaminyl-L-leucyl-L-methionyl-L-histidyl-L-asparaginyl-L-leucylglycyl-L-lysyl-L-histidyl-L-leucyl-L-asparaginyl-L-seryl-L-methionyl-L-alpha-glutamyl-L-arginyl-L-valyl-L-alpha-glutamyl-L-tryptophyl-L-leucyl-L-arginyl-L-lysyl-L-lysyl-L-leucyl-L-glutaminyl-L-alpha-aspartyl-L-valyl-L-histidyl-L-asparaginyl-, acetate (salt) hydrate
- L-Seryl-L-valyl-L-seryl-L-alpha-glutamyl-L-isoleucyl-L-glutaminyl-L-leucyl-L-methionyl-L-histidyl-L-asparaginyl-L-leucylglycyl-L-lysyl-L-histidyl-L-leucyl-L-asparaginyl-L-seryl-L-methionyl-L-alpha-glutamyl-L-arginyl-L-valyl-L-alpha-glutamyl-L-tryptophyl-L-leucyl-L-arginyl-L-lysyl-L-lysyl-L-leucyl-L-glutaminyl-L-alpha-aspartyl-L-valyl-L-histidyl-L-asparaginyl-L-phenylalanine acetate (salt) hydrate
- Parathar
- Parathar acetate
- L-Phenylalanine, L-seryl-L-valyl-L-seryl-L-alpha-glutamyl-L-isoleucyl-L-glutaminyl-L-leucyl-L-methionyl-L-histidyl-L-asparaginyl-L-leucylglycyl-L-lysyl-L-histidyl-L-leucyl-L-asparaginyl-L-seryl-L-methionyl-L-alpha-glutamyl-L-arginyl-L-valyl-L-alpha-glutamyl-L-tryptophyl-L-leucyl-L-arginyl-L-lysyl-L-lysyl-L-leucyl-L-glutaminyl-L-alpha-aspartyl-L-valyl-L-histidyl-L-asparaginyl-, acetate (salt)
Teriparatide is a synthetic form of parathyroid hormone that is used to treat osteoporosis in postmenopausal women. It has been shown to increase bone mineral density and reduce the risk of fractures. Teriparatide acetate, a new formulation of teriparatide, has been found to be more effective than the current formulation. This medication can also be used for patients with bowel disease and body mass index between 25-50 kg/m2. Teriparatide acetate may be less likely to cause side effects than other forms of teriparatide because it is given as an injection rather than by mouth. The use of teriparatide acetate in clinical trials has been approved by the FDA for the treatment of osteoporosis in postmenopausal women who have been diagnosed with inflammatory bowel disease or who cannot take oral medications due to bowel disease or other conditions. Teriparatide acetate is administered intravenously over five minutes and should not be