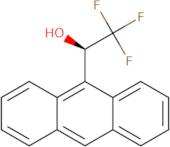
(R)-(-)-2,2,2-Trifluoro-1-(9-Anthryl)Ethanol
CAS: 53531-34-3
Ref. 3D-FT88356
| 1g | Discontinued | ||
| 2g | Discontinued |
Product Information
- (-)-1-(9-Anthryl)-2,2,2-trifluoroethanol
- (-)-alpha-(Trifluoromethyl)-9-anthracenemethanol
- (1R)-1-(9-Anthryl)-2,2,2-trifluoroethanol
- (1R)-1-anthracen-9-yl-2,2,2-trifluoroethanol
- (Minus)-2,2,2-Trifluoro-1-(9-Anthryl)-*Ethanol
- (R)-(-)-1-(9-Anthryl)-2,2,2-trifluoroethanol
- (R)-(-)-2,2,2-Trifluoro-1-(9-anthryl)ethanol
- (R)-1-(9-Anthryl)-2,2,2-trifluoro-1-ethanol
- (R)-1-(Anthracen-10-yl)-2,2,2-trifluoroethanol
- (R)-1-(Anthracen-9-yl)-2,2,2-trifluoroethanol
- See more synonyms
- (R)-2,2,2-Trifluoro-1-(9-anthracenyl)ethanol
- (αR)-α-(Trifluoromethyl)-9-anthracenemethanol
- 1-(Anthracen-9-Yl)-2,2,2-Trifluoroethanol
- 9-Anthracenemethanol, α-(trifluoromethyl)-, (R)-
- 9-Anthracenemethanol, α-(trifluoromethyl)-, (αR)-
- (-)-2,2,2-Trifluoro-1-(9-anthryl)ethanol
(R)-(-)-2,2,2-Trifluoro-1-(9-anthryl)ethanol (TFAE) is a chiral organic molecule that can be used as a synthetic intermediate in the synthesis of other compounds. This compound is an enantiomer of 2,2,2-trifluoro-1-(9-anthryl)ethanol (TFAE), which has been synthesized by the hydrogenation of TFAE. TFAE is thermodynamically stable and can be purified by chromatographic methods. It has also been shown to have antiinflammatory properties. The asymmetric synthesis of this compound was achieved using ethyl esters, which are more expensive than methyl esters. However, the use of ethyl esters led to the production of higher yields and better stereoselectivity than when using methyl esters. A molecular modeling study was conducted on this compound to determine its structural elucidation and confirm its configuration