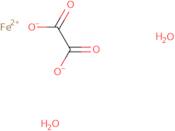
Product Information
- (T-4)-Diaqua[ethanedioato(2-)-κO<sup>1</sup>,κO<sup>2</sup>]iron
- Ethanedioic acid, iron(2+) salt (1:1), dihydrate
- Ferrous Oxalate
- Ferrous Oxalate Dihydrate
- Ferrous oxalate (FeC<sub>2</sub>O<sub>4</sub>) dihydrate
- Iron oxalate (FeC<sub>2</sub>O<sub>4</sub>) dihydrate
- Iron oxalate hydrate (FeC<sub>2</sub>O<sub>4</sub>.2H<sub>2</sub>O)
- Iron(2+) Ethanedioate Hydrate (1:1:2)
- Iron(2+) oxalate dihydrate
- Iron, diaqua(ethanedioato(2-)-kappao1,kappao2)-, (T-4)-
- See more synonyms
- Iron, diaqua[ethanedioato(2-)-O,O′]-
- Iron, diaqua[ethanedioato(2-)-κO<sup>1</sup>,κO<sup>2</sup>]-, (T-4)-
- Ironoxalatedihydrateminyellowpowder
- Oxalic acid, iron(2+) salt (1:1), dihydrate
- Iron (II) oxalate dihydrate
Iron(II)oxalatedi hydrate is a model system that can be used to study the reaction mechanism of the oxidation of hydrocarbons. It is created by forming an iron oxalate salt from iron(III) oxide and oxalic acid. The reaction solution contains a cationic surfactant, which stabilizes the particle in solution. The protonated form of the oxalatedi hydrate has a kinetic rate constant of 1.4 x 10^-5 s^-1 at 25°C and pH 7, with a structural analysis showing that this particle is composed of a lithium ion surrounded by four oxalates and two water molecules. This particle reacts with hydrocarbons to produce carbon dioxide and hydrogen gas, which are then oxidized to form water and carbon monoxide.
Chemical properties
Technical inquiry about: 3D-GAA04725 Iron(II) oxalate dihydrate
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.