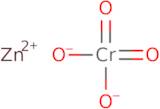
Zinc chromate
CAS: 13530-65-9
Ref. 3D-NAA53065
| 1kg | Discontinued | ||
| 5kg | Discontinued | ||
| 10kg | Discontinued | ||
| 250g | Discontinued | ||
| 500g | Discontinued |
Product Information
- Buttercup yellow
- Chromate de zinc
- Chromic acid (H<sub>2</sub>CrO<sub>4</sub>), zinc salt (1:1)
- Chromium zinc oxide (ZnCrO4)
- Chromium zinc oxide (ZnCrO<sub>4</sub>)
- Cromato De Cinc
- Zinc Chromate
- Zinc chromate (Zinc chromate hydroxide)
- Zinc chromate (ZnCrO4)
- Zinc chromate (ZnCrO<sub>4</sub>)
- See more synonyms
- Zinc chromate(VI) (ZnCrO4)
- Zinc chromate(VI) (ZnCrO<sub>4</sub>)
- Zinc chrome yellow
- Zinc chromium oxide (ZnCrO4)
- Zinc chromium oxide (ZnCrO<sub>4</sub>)
- Zincro ZTO
- Zinkchromat
- Zinkchromat(Vi)
- Chromic acid (H2CrO4), zinc salt (1:1)
- C.I. 77955
- zinc dioxido(dioxo)chromium
Zinc chromate is a chemical compound that is used in the production of paints, dyes, and pigments. It is also used to coat metal surfaces to prevent corrosion and rusting. Zinc chromate can be found in wastewater effluent from the electroplating industry. Chronic exposure to zinc chromate may result in particle deposition in the bronchial tree and lungs. In addition, it has carcinogenic potential and has been shown to cause cancer in animals. Zinc chromate is synthesized by combining potassium dichromate with zinc oxide or zirconium oxide. Zinc chromate can be synthesized by reacting copper chloride with an alkaline solution of sodium dichromate or calcium hydroxide, followed by adding a fatty acid such as stearic acid or oleic acid. The reaction produces zinc hydroxychloride, which reacts with hydrogen chloride gas to form zinc chloride and hydrogen hydrochloride gas. The product of this reaction is then heated at high