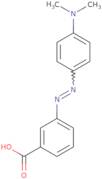
m-Methyl Red
CAS: 20691-84-3
Ref. 3D-VAA69184
| 1g | To inquire | ||
| 5g | To inquire | ||
| 10g | To inquire | ||
| 25g | To inquire | ||
| 500mg | To inquire |
Product Information
- 3-[2-[4-(Dimethylamino)phenyl]diazenyl]benzoic acid
- 3-[4-(Dimethylamino)phenylazo]benzoic acid
- 3-[[4-(Dimethylamino)phenyl]diazenyl]benzoic acid
- 3-{(E)-[4-(dimethylamino)phenyl]diazenyl}benzoic acid
- 3′-Carboxy-4-dimethylaminoazobenzene
- Benzoic acid, 3-[2-[4-(dimethylamino)phenyl]diazenyl]-
- Benzoic acid, 3-[[4-(dimethylamino)phenyl]azo]-
- Benzoic acid, m-[[p-(dimethylamino)phenyl]azo]-
- Meta-methyl red
Phenolphthalein is a tautomeric molecule that can be found as the acid form (phenolphthalein) or the salt form (m-methyl red). It is an acidic, colorless compound and has two functional groups: the phenol and the thiocyanate. Phenolphthalein changes its color from clear to pink when it is exposed to an alkali. The change in color occurs because of hydrogen bonding, which is also responsible for some of phenolphthalein's other properties. When phenolphthalein is dissolved in water, it forms a complex with bromocresol purple that absorbs light at a different wavelength than phenolphthalein alone. This property makes it useful for testing for substances that are acidic or basic.
Methyl Red has been used extensively as a pH indicator in biochemistry research and teaching laboratories since its introduction by J.P. Greenstein in 1968. It changes color from yellow to red
Chemical properties
Technical inquiry about: 3D-VAA69184 m-Methyl Red
If you want to request a quotation or place an order, please instead add the desired products to your cart and then request a quotation or order from the cart. It is faster, cheaper, and you will be able to benefit from the available discounts and other advantages.