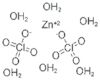
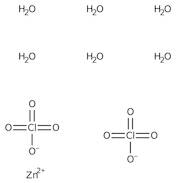
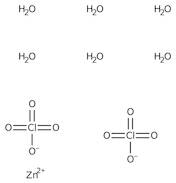
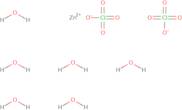
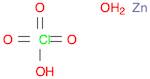
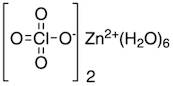CAS: 10025-64-6 - Zinc perchlorate hexahydrate
Formula:Cl2O8Zn
InChI:InChI=1S/ClHO4.3H2O.Zn/c2-1(3,4)5;;;;/h(H,2,3,4,5);3*1H2;
InChI key:InChIKey=ZMHJVBYCGCPUEW-UHFFFAOYSA-N
SMILES:[Zn].O=Cl(=O)(=O)O.O
- Synonyms:
- Perchloric acid, zinc salt, hexahydrate
- Perchloric acid, zinc salt, hydrate (2:1:6)
- Zinc Diperchlorate
- Zinc Perchlorate Hydrate (1:2:6)
- Zinc diperchlorate hexahydrate
- Zinc perchlorate (Zn(ClO<sub>4</sub>)<sub>2</sub>) hexahydrate
- Zinc perchlorate hexahydrate (Zn(ClO<sub>4</sub>)<sub>2</sub>.6H<sub>2</sub>O)

Zinc perchlorate hexahydrate, 99.997% (metals basis)
CAS:10025-64-6
Used as intermediates. A synthetic reagent for proteomics research. Zinc(II) perchlorate hexahydrate is a highly …
Formula:
Cl2H12O14Zn
Purity:
99.997%
Color and Shape:
White Solid Crystalline
Molecular weight:
372.36
Ref: 02-044315
| 10g | 94.00 € | |
| 50g | 319.00 € |
Estimated delivery in United States, on Friday 3 May 2024

Zinc perchlorate hexahydrate, Reagent Grade
CAS:10025-64-6
Zinc perchlorate hexahydrate, efficiently catalyses the esterification between nearly equimolar amounts of carboxylic acids and …
Formula:
Cl2H12O14Zn
Color and Shape:
Crystalline
Molecular weight:
372.36
Ref: 02-011614
| 100g | 57.00 € | |
| 500g | 183.00 € |
Estimated delivery in United States, on Friday 3 May 2024

Zinc perchlorate hexahydrate, 99%
CAS:10025-64-6
Formula:
Cl2O8Zn
Purity:
99%
Color and Shape:
white xtl.
Molecular weight:
264.27 (372.36)
Ref: 08-93-3018
| 100g | 38.00 € | |
| 500g | 130.00 € |
Estimated delivery in United States, on Friday 10 May 2024

Zinc perchlorate hexahydrate
CAS:10025-64-6
Zinc perchlorate hexahydrate is a nucleophilic, organometallic compound that is used as a reagent in …
Formula:
ZnCl2O14H12
Purity:
Min. 95%
Molecular weight:
372.37 g/mol
Ref: 3D-FZ76241
| 25g | To inquire | |
| 50g | To inquire | |
| 100g | To inquire |
Estimated delivery in United States, on Thursday 20 Jun 2024

Perchloric acid, zinc salt, hydrate (2:1:6)
CAS:10025-64-6
Formula:
Cl2H12O14Zn
Purity:
99%
Color and Shape:
Solid
Molecular weight:
372.3729
Ref: AN-AG0001DW
| 5g | Discontinued | |
| 25g | Discontinued |