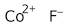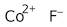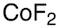Cobalt(II) fluoride, anhydrous, 98%
CAS:10026-17-2
Cobalt(II) fluoride can be used as a catalyst to alloy metals. It is also used …
Formula:
CoF2
Purity:
98%
Color and Shape:
Powder
Molecular weight:
96.93
Ref: 02-013074
| 25g | 78.00 € | |
| 100g | 213.00 € |
Estimated delivery in United States, on Monday 6 May 2024

Cobalt(II) fluoride, ultra dry, 99.99% (metals basis)
CAS:10026-17-2
It is used as a catalyst to alloy metals and also used for optical deposition, …
Formula:
CoF2
Purity:
99.99%
Color and Shape:
Powder, Pink to red
Molecular weight:
96.93
Ref: 02-035740
| 2g | 81.00 € | |
| 10g | 238.00 € |
Estimated delivery in United States, on Monday 6 May 2024

Cobalt difluoride, anhydrous
CAS:10026-17-2
Purity:
99.0%
Color and Shape:
Solid
Molecular weight:
96.93000030517578
Ref: 10-F004570
| 1g | 36.00 € | |
| 25g | 178.00 € | |
| 100g | 475.00 € |
Estimated delivery in United States, on Tuesday 7 May 2024

Cobalt(II) fluoride, anhydrous, 99%
CAS:10026-17-2
Formula:
CoF2
Purity:
99%
Color and Shape:
pink pwdr.
Molecular weight:
96.93
Ref: 08-93-2738
| 10g | 58.00 € | |
| 50g | 187.00 € |
Estimated delivery in United States, on Monday 13 May 2024

Cobalt(II) Fluoride
CAS:10026-17-2
Formula:
CoF2
Color and Shape:
Light red to Red to Purple powder to crystal
Molecular weight:
96.93
Ref: 3B-C3752
| 25g | 82.00 € | |
| 100g | 230.00 € |
Estimated delivery in United States, on Monday 13 May 2024

Cobalt(II) fluoride, anhydrous
CAS:10026-17-2
Purity:
99%
Color and Shape:
Pale Pink Powder
Molecular weight:
96.93g/mol
Ref: 54-PC2080
| 1g | 21.00 € | |
| 25g | 122.00 € | |
| 100g | 194.00 € |
Estimated delivery in United States, on Friday 17 May 2024