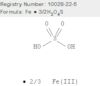
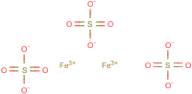
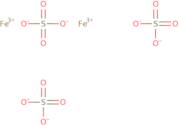
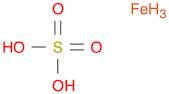
CAS: 10028-22-5 - Sulfuric acid, iron(3+) salt (3:2)
Formula:Fe.3/2H2O4S
InChI:InChI=1S/Fe.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)
InChI key:InChIKey=MVZXTUSAYBWAAM-UHFFFAOYSA-N
SMILES:[Fe].O=S(=O)(O)O
- Synonyms:
- Amersep 5320
- Dieisentris(sulfat)
- Diiron Tris(Sulfate)
- Diiron Tris(Sulphate)
- Diiron trisulfate
- Diiron(3+) sulfate
- FS-Pix
- Ferric Sulfate
- Ferric sulphate
- Iron (Iii) Sulfate
- See more synonyms
- Iron persulfate
- Iron sesquisulfate
- Iron sulfate (2:3)
- Iron sulfate (Fe2(SO4)3)
- Iron sulfate (Fe<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>)
- Iron(3+) sulfate
- Pix 112
- Pix 115
- Pix 123
- Stat Gel
- Tris(Sulfato) De Dihierro
- Tris(sulfate) de difer
- ViscoStat
| Brand | Product data | Purity | Price range | Estimated delivery |
|---|---|---|---|---|
 | Iron (III) Sulfate REF: 54-IN2090CAS: 10028-22-5 | reagent grade | To inquire | Tue 21 May 24 |
 | Iron(III) sulfate REF: 3D-FI47136CAS: 10028-22-5 | - - - | 104.00 €~496.00 € | Tue 28 May 24 |
 | Sulfuric acid, iron(3+) salt (3:2) REF: AN-AG0001GJCAS: 10028-22-5 | 99% | 25.00 € | Fri 17 May 24 |

Iron (III) Sulfate
CAS:10028-22-5
Purity:
reagent grade
Color and Shape:
Yellow Solid
Molecular weight:
399.88g/mol
Ref: 54-IN2090
| Undefined size | To inquire |
Estimated delivery in United States, on Tuesday 21 May 2024

Iron(III) sulfate
CAS:10028-22-5
Iron(III) sulfate is a chemical compound that is widely used in the water treatment industry. …
Formula:
Fe2O12S3
Color and Shape:
Powder
Molecular weight:
405.93 g/mol
Ref: 3D-FI47136
| 1kg | 165.00 € | |
| 2kg | 267.00 € | |
| 5kg | 362.00 € | |
| 10kg | 496.00 € | |
| 500g | 104.00 € |
Estimated delivery in United States, on Tuesday 28 May 2024

Sulfuric acid, iron(3+) salt (3:2)
CAS:10028-22-5
Formula:
Fe2O12S3
Purity:
99%
Color and Shape:
Solid
Molecular weight:
399.8778
Ref: AN-AG0001GJ
| 25g | Discontinued |