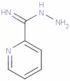
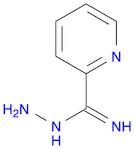
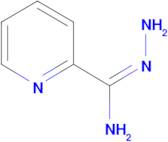
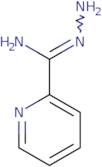
CAS: 1005-02-3 - pyridine-2-carboximidohydrazide
Formula:C6H8N4
InChI:InChI=1S/C6H8N4/c7-6(10-8)5-3-1-2-4-9-5/h1-4H,8H2,(H2,7,10)
InChI key:InChIKey=DKTIHEQAQFSEAB-UHFFFAOYSA-N
SMILES:N=C(NN)C1=NC=CC=C1
- Synonyms:
- 2-Picolinamidrazone
- 2-Pyridine amidrazone
- 2-Pyridinecarboxamidrazone
- 2-Pyridinecarboximidic acid, hydrazide
- 2-Pyridylamidrazone
- NSC 101642
- Picolinamide hydrazone
- Picolinamidrazone
- Picolinic acid amidrazone
- Picolinimidic acid, hydrazide
- See more synonyms
- Pyridine-2-Carbohydrazonamide
- Pyridine-2-carboxamide hydrazone
- Pyridine-2-hydrazidine
| Brand | Product data | Purity | Price range | Estimated delivery |
|---|---|---|---|---|
 | 2-Pyridinecarboximidic acid, hydrazide REF: AN-AG000259CAS: 1005-02-3 | 97%;RG | 41.00 €~515.00 € | Wed 15 May 24 |
 | Picolinimidohydrazide REF: 10-F412000CAS: 1005-02-3 | 98% | To inquire | Tue 28 May 24 |
 | N-Aminopyridine-2-carboximidamide REF: 3D-BAA00502CAS: 1005-02-3 | Min. 95% | To inquire | Wed 26 Jun 24 |

2-Pyridinecarboximidic acid, hydrazide
CAS:1005-02-3
Formula:
C6H8N4
Purity:
97%;RG
Color and Shape:
Solid
Molecular weight:
136.1545
Ref: AN-AG000259
| 1g | 63.00 € | |
| 5g | 191.00 € | |
| 25g | 515.00 € | |
| 250mg | 53.00 € |
Estimated delivery in United States, on Wednesday 15 May 2024

Ref: 10-F412000
| 1g | To inquire | |
| 5g | To inquire | |
| 25g | To inquire |
Estimated delivery in United States, on Tuesday 28 May 2024

N-Aminopyridine-2-carboximidamide
CAS:1005-02-3
N-Aminopyridine-2-carboximidamide (NAP) is a potent inhibitor of the β-catenin pathway. It is used to treat …
Formula:
C6H8N4
Purity:
Min. 95%
Molecular weight:
136.15 g/mol
Ref: 3D-BAA00502
| 1g | To inquire | |
| 5g | To inquire | |
| 10g | To inquire | |
| 25g | To inquire | |
| 500mg | To inquire |
Estimated delivery in United States, on Wednesday 26 Jun 2024