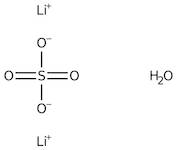
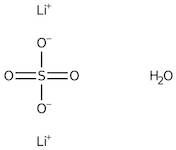
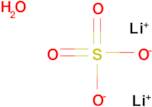
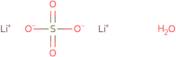
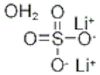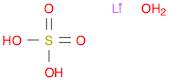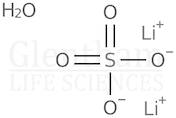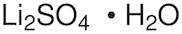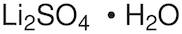CAS: 10102-25-7 - Lithium sulfate monohydrate
Formula:H2LiO5S
InChI:InChI=1S/2Li.H2O4S.H2O/c;;1-5(2,3)4;/h;;(H2,1,2,3,4);1H2
InChI key:InChIKey=YXUBOVYBMFWYOG-UHFFFAOYSA-N
SMILES:[Li].O=S(=O)(O)O.O
- Synonyms:
- Dilithium sulfate monohydrate
- Lithium Sulfate Hydrate
- Lithium Sulfate Hydrate (2:1:1)
- Lithium Sulphate Monohydrate
- Lithium mesosulfate
- Lithium sulfate (Li<sub>2</sub>SO<sub>4</sub>) monohydrate
- Lithium sulfate momohydrate
- Peramin AXL 80
- Sulfuric acid, dilithium salt, monohydrate
- Sulfuric acid, lithium salt, hydrate (1:2:1)
- See more synonyms

Lithium sulfate monohydrate, 99%
CAS:10102-25-7
Lithium sulfate is used to treat bipolar disorder. It is also used in ultrasound-type non-destructive …
Formula:
H2Li2O5S
Purity:
99%
Color and Shape:
White, Crystalline powder
Molecular weight:
127.95
Ref: 02-A10410
| 100g | 25.00 € | |
| 500g | 86.00 € | |
| 2500g | 466.00 € |
Estimated delivery in United States, on Thursday 9 May 2024

Lithium sulfate monohydrate, ACS, 99.0% min
CAS:10102-25-7
Lithium sulfate monohydrate is used in solar panels and potential for a new class of …
Formula:
H2Li2O5S
Purity:
99.0%
Color and Shape:
White Powder Solid
Molecular weight:
127.95
Ref: 02-036216
| 2kg | 458.00 € | |
| 100g | 51.00 € | |
| 500g | 136.00 € |
Estimated delivery in United States, on Thursday 9 May 2024

Sulfuric acid, lithium salt, hydrate (1:2:1)
CAS:10102-25-7
Formula:
H2Li2O5S
Purity:
99%
Color and Shape:
Solid
Molecular weight:
127.9599
Ref: AN-AG0003K1
| 25g | 25.00 € | |
| 100g | 34.00 € | |
| 500g | 86.00 € | |
| 1000g | 139.00 € |
Estimated delivery in United States, on Wednesday 15 May 2024

Lithium sulfate monohydrate, 99%
CAS:10102-25-7
Formula:
Li2SO4·H2O
Purity:
≥ 99.0% (Li2SO4 · H2O)
Color and Shape:
White crystalline powder or colourless crystals
Molecular weight:
127.95
Ref: 7W-GX2584
| 1kg | 130.00 € | |
| 100g | 26.00 € | |
| 500g | 80.00 € |
Estimated delivery in United States, on Thursday 16 May 2024

Lithium sulphate monohydrate
CAS:10102-25-7
Purity:
99+%
Color and Shape:
Solid
Molecular weight:
127.96g/mol
Ref: 54-IN2360
| 500g | 93.00 € |
Estimated delivery in United States, on Thursday 16 May 2024

Lithium sulfate, monohydrate, 99.9+%
CAS:10102-25-7
Formula:
Li2SO4·H2O
Purity:
≥ 99.9%
Color and Shape:
White powder or crystals
Molecular weight:
127.95
Ref: 7W-GX7464
| 50g | 218.00 € |
Estimated delivery in United States, on Wednesday 22 May 2024

Ref: 10-F494106
| 1kg | To inquire | |
| 100g | To inquire | |
| 250g | 71.00 € | |
| 2.5kg | To inquire |
Estimated delivery in United States, on Tuesday 28 May 2024

Lithium Sulphate Monohydrate extrapure AR, ACS, ExiPlus, Multi-Compendial, 99%
CAS:10102-25-7
Formula:
Li2SO4·H2O
Purity:
min. 99%
Color and Shape:
White, Crystalline compound, Clear, Colourless
Molecular weight:
127.96
Ref: SR-39478
| 100g | 48.00 € | |
| 250g | 102.00 € | |
| 500g | 200.00 € |
Estimated delivery in United States, on Wednesday 12 Jun 2024

Lithium Sulphate Monohydrate extrapure AR, 99%
CAS:10102-25-7
Formula:
Li2SO4·H2O
Purity:
min. 99% (dried basis )
Color and Shape:
White, Crystalline compound, Clear, Colourless
Molecular weight:
127.96
Ref: SR-88181
| 100g | 45.00 € | |
| 250g | 98.00 € | |
| 500g | 188.00 € |
Estimated delivery in United States, on Wednesday 12 Jun 2024

Lithium sulfate monohydrate - ACS
CAS:10102-25-7
Lithium sulfate monohydrate is a lithium salt that is used as a reagent to produce …
Formula:
Li2SO4·H2O
Purity:
Min. 95%
Color and Shape:
Solid
Molecular weight:
127.96 g/mol
Ref: 3D-FL54607
| 1kg | To inquire | |
| 2kg | To inquire | |
| 5kg | To inquire | |
| 250g | To inquire | |
| 500g | To inquire |
Estimated delivery in United States, on Wednesday 26 Jun 2024