Pharmaceutical Standards
Pharmaceutical standards are a comprehensive set of reference materials essential for ensuring the safety, efficacy, and quality of pharmaceutical products. This category includes standards for active pharmaceutical ingredients (APIs), which are the core components responsible for therapeutic effects. Additionally, it covers compounds and metabolites relevant to both the pharmaceutical and veterinary industries, providing benchmarks for the accurate measurement and analysis of these substances. Nitrosamine control standards are crucial for detecting and mitigating potentially harmful nitrosamines in drug formulations. Toxicology standards help assess the safety and potential adverse effects of pharmaceutical compounds. Furthermore, enzyme activators and inhibitors standards are vital for research and development, enabling precise studies of biochemical pathways and drug mechanisms. These pharmaceutical standards are indispensable tools for regulatory compliance, quality control, and research, ensuring that pharmaceutical products meet stringent safety and effectiveness criteria.
Subcategories of "Pharmaceutical Standards"
Products of "Pharmaceutical Standards"
| Brand | Product data | Purity | Price range | Estimated delivery |
|---|---|---|---|---|
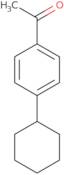
 | 4'-Cyclohexylacetophenone REF: 3D-FC70867CAS: 18594-05-3 | Min. 95% | - - - | Discontinued product |
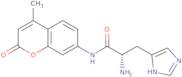
 | L-Histidine 7-amido-4-methylcoumarin REF: 3D-FH48733CAS: 191723-64-5 | Min. 99 Area-% | - - - | Discontinued product |

4'-Cyclohexylacetophenone
Ref: 3D-FC70867
| 1kg | Discontinued | Request information | |
| 2kg | Discontinued | Request information | |
| 100g | Discontinued | Request information | |
| 250g | Discontinued | Request information | |
| 500g | Discontinued | Request information |

L-Histidine 7-amido-4-methylcoumarin
Ref: 3D-FH48733
| 10mg | Discontinued | Request information | |
| 25mg | Discontinued | Request information | |
| 50mg | Discontinued | Request information |