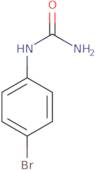
(4-Bromophenyl)urea
CAS: 1967-25-5
Ref. 3D-FB61339
| 5g | Discontinued | ||
| 10g | Discontinued | ||
| 25g | Discontinued | ||
| 50g | Discontinued | ||
| 100g | Discontinued |
Product Information
- Urea, (4-bromophenyl)-
- Ai3-61301
- 1-(4-Bromophenyl)Urea
The reaction of trimethyl bromide with chlorate in an organic solvent is a nucleophilic substitution reaction. The reaction rate increases with the concentration of the reactants and decreases with temperature. The mechanism of the reaction is similar to that of nitrous acid. One proton transfers from the bromide to the chloride ion, forming a peroxide intermediate, which reacts with water to give ethylene glycol. This product is then hydrolysed by hydroxide ions to form hydrogen peroxide, and this reacts with dihydroxybenzene (chlorobenzene) to produce 4-bromophenyl urea.