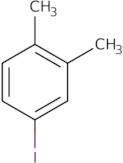
4-Iodo-1,2-dimethylbenzene
CAS: 31599-61-8
Ref. 3D-FI137550
| 10g | Discontinued | ||
| 25g | Discontinued | ||
| 50g | Discontinued | ||
| 100g | Discontinued | ||
| 250g | Discontinued |
Product Information
- (4-Formyl-5-hydroxy-6-methyl-3-pyridinyl)methyl dihydrogen phosphate
- (4-Formyl-5-hydroxy-6-methyl-3-pyridinyl)methyldihydrogen-phosphat
- (4-Formyl-5-hydroxy-6-methylpyridin-3-yl)methyl dihydrogen phosphate
- (4-Formyl-5-hydroxy-6-methylpyridin-3-yl)methyl-dihydrogen-_phosphat
- (4-Formyl-5-hydroxy-6-methylpyridin-3-yl)methyldihydrogen-phosphat
- 1,2-Dimethyl-4-iodobenzene
- 1-Iodo-3,4-dimethylbenzene
- 200-208-3
- 3,4-Dimethylphenyl iodide
- 4-Iodo-1,2-Dimethylbenzene
- See more synonyms
- 4-Iodo-o-xylene
- 4-Pyridinecarboxaldehyde, 3-hydroxy-2-methyl-5-[ (phosphonooxy)methyl]-
- Aderoxal
- Benzene, 4-iodo-1,2-dimethyl-
- o-Xylene, 4-iodo-
4-Iodo-1,2-dimethylbenzene (4IDMB) is an arylate that can be used as a toolbox. It is used in the synthesis of glycopeptide antibiotics and other drugs by reductive elimination of 4IDMB to form an aryl group. The phenyl groups provide reactive sites for reactions with other molecules such as acetoxylation, carbon-carbon bond formation, alkylation, and phthaloylation. The most common use for 4IDMB is in the synthesis of penicillins by acetoxylation followed by alkylation. 4IDMB has been shown to react at room temperature with picolinic acid to form a diastereomer. This reaction can also be carried out at higher temperatures to produce a different diastereomer. As an example, the reaction with picolinic acid can be carried out at refluxing temperatures to produce the desired diastereomer due to