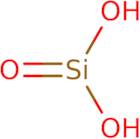
Silicic acid
CAS: 1343-98-2
Ref. 3D-FS106437
| 10g | Discontinued | ||
| 25g | Discontinued | ||
| 50g | Discontinued | ||
| 100g | Discontinued | ||
| 250g | Discontinued |
Product Information
- 200Fad
- Acide silicique
- Acido Silicico
- Adsolider 102
- Adsorder 101
- Aeroperl 90/30
- Betol
- Betol (acid)
- Cubosic
- Ds 100
- See more synonyms
- Ds 200
- Euderm Duller SN 2
- H-Ilerit
- Hydrosilicic acid
- K 320Ds
- K 60
- K 60 (silicate)
- Kieselsaure
- Mikronisil
- Neoxyl ET
- Nevsil 115
- Nipsil AQ-S
- Polyorthosilicic acid
- Polysilicic acid
- Silica acid
- Silica gel
- Silicagel
- Silicea
- Silicic acid hydrate
- Silicon hydroxide
- Silton TF 06
- Sipernat 17
- Sipernat 320DS
- Sipernat 350
- Sipernat 500DS
- Sipernat S
- Sizol 0-30
- Tetrahydroxysilane homopolymer
- Tetrahydroxysilane polymer
- Tokusil PR
- Tokusil U 15
- Vulcasil S/GR
- Zeosil 500V
Silicic acid is a mineral that occurs naturally in the earth's crust. It is used for the treatment of wastewater and industrial effluents due to its high adsorption capacity and chemical stability. Silicic acid is an ionized, acidic compound with a redox potential of -1.8 V, which can be used as an alternative to hydrochloric acid in biological treatment systems. The uptake mechanism of silicic acid is not well understood, but it has been observed to occur via membrane channels and electrostatic interactions with negatively charged particles. The uptake rate of silicic acid depends on particle size and concentration, with higher concentrations leading to greater uptake rates. Silica is also used in the production of many products such as glass, ceramics, and silicon chips.