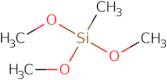
Trimethoxymethylsilane
CAS: 1185-55-3
Ref. 3D-FT54905
| 1kg | Discontinued | ||
| 2kg | Discontinued | ||
| 5kg | Discontinued | ||
| 10kg | Discontinued | ||
| 500g | Discontinued |
Product Information
- (Trimethoxysilyl)methane
- A 163
- A 1630
- Ay 43-043
- Cg 68
- Cg 8030
- Cg-N 113
- D 30 (silane)
- Db 8030
- Dmt 20
- See more synonyms
- Dsl 9900
- Dynasylan MTMS
- Glasca B
- Jh-N 311
- Kbm 11
- Kbm 13
- Kc 89R
- Kh 131
- Kh 163
- Kh 370
- LS 530 (silane)
- Lmd 921
- Ls 530
- M 9100
- MTMS (silane)
- Methyl-Trimethoxy-Silan
- Methylsilicon trimethoxide (MeSi(OMe)3)
- Methylsilicon trimethoxide (MeSi(OMe)<sub>3</sub>)
- Methyltrimethoxysilane
- Methyltrimethyloxysilane
- Metiltrimetoxisilano
- Mts 31
- N 113
- NUC Silicone A 163
- Nd 28
- Np 31
- Nsc 93883
- Ofs 6070
- Si 1630
- Si 41
- Silane M 1 Trimethoxy
- Silquest A 163
- Silquest A 1630
- Silquest A 1630A
- Sim 6560.0
- Sip 6560.0
- Ss 1670
- Sz 6070
- Trimethoxy(methyl)silan
- Trimethoxy(methyl)silane
- Tsl 8113
- Up 302
- Wd 921
- We 22
- Xiameter OFS 6070
- Xinlantian D 30
- Z 6070
- Z 6366
- Zh 1101
- Silane, trimethoxymethyl-
- Methytrimethoxysilane
- MTMS
Trimethoxymethylsilane is a chemical compound that is used to form a hydrophobic surface on zirconium oxide. It is commonly used in the production of dental prostheses and surgical implants. The reaction solution for this process contains sodium carbonate, which reacts with trimethoxymethylsilane and water to form sodium methanolate, which reacts with zirconium oxide particles to form a hydrophobic surface. Trimethoxymethylsilane has been shown to be chemically stable in human serum and methanol solutions. The gel pores formed by this compound are smaller than those formed by other similar compounds, such as octyltrimethoxysilane or hexamethyldisilazane.