Compostos alifáticos e derivados
Os compostos alifáticos e seus derivados são compostos orgânicos caracterizados por estruturas de cadeia reta ou ramificada, ao contrário das estruturas em anel encontradas nos compostos aromáticos. Esses compostos incluem alcanos, alcenos, alcinos e seus derivados funcionalizados, desempenhando um papel vital em vários processos químicos e aplicações industriais. Na CymitQuimica, oferecemos uma seleção diversificada de compostos alifáticos de alta pureza e seus derivados, meticulosamente selecionados e testados para atender aos rigorosos requisitos de pesquisa e necessidades industriais. Nosso catálogo abrange uma ampla gama de compostos, incluindo hidrocarbonetos, álcoois, aldeídos, cetonas e ácidos, cada um conhecido por sua reatividade e versatilidade na síntese orgânica, farmacêutica e ciência dos materiais. Ao fornecer compostos alifáticos e derivados de alta qualidade, apoiamos pesquisadores e profissionais na realização de transformações químicas precisas e eficientes, promovendo a inovação e os avanços em vários campos científicos e tecnológicos.

ethyl 4-[(E)-2-(dimethylamino)vinyl]-8-(4-fluorophenyl)pyrazolo[5,1-c][1,2,4]triazine-3-carboxylate
Ref: 10-F371590
| 1g | Descontinuado | Solicitar informações | |
| 5g | Descontinuado | Solicitar informações |

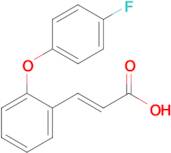
(E)-3-(2-(4-fluorophenoxy)phenyl)acrylic acid
Ref: 10-F773099
| 1g | Descontinuado | Solicitar informações | |
| 5g | Descontinuado | Solicitar informações |

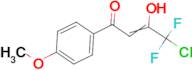
4-Chloro-4,4-difluoro-1-(4-methoxy-phenyl)-butane-1,3-dione
Ref: 10-F026078
| 1g | Descontinuado | Solicitar informações | |
| 2.5g | Descontinuado | Solicitar informações | |
| 50mg | Descontinuado | Solicitar informações | |
| 100mg | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

Ref: 10-F752699
| 5g | Descontinuado | Solicitar informações | |
| 25g | Descontinuado | Solicitar informações |

Ref: 10-F673454
| 1g | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

Ref: 10-F715772
| 1g | Descontinuado | Solicitar informações | |
| 5g | Descontinuado | Solicitar informações |

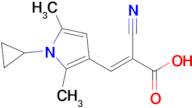
(E)-2-Cyano-3-(1-cyclopropyl-2,5-dimethyl-1H-pyrrol-3-yl)acrylic acid
Ref: 10-F644331
| 1g | Descontinuado | Solicitar informações | |
| 50mg | Descontinuado | Solicitar informações | |
| 100mg | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

Ref: 10-F722392
| 1g | Descontinuado | Solicitar informações | |
| 5g | Descontinuado | Solicitar informações | |
| 10g | Descontinuado | Solicitar informações | |
| 50mg | Descontinuado | Solicitar informações | |
| 100mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

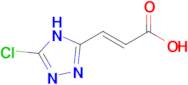
(2E)-3-(5-chloro-4H-1,2,4-triazol-3-yl)prop-2-enoic acid
Ref: 10-F768368
| 1g | Descontinuado | Solicitar informações | |
| 5g | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações |

Ref: 10-F677589
| 1g | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

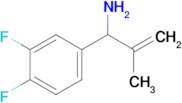
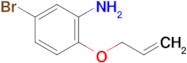
1-(3,4-Difluorophenyl)-2-methylprop-2-en-1-amine
Ref: 10-F677458
| 1g | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

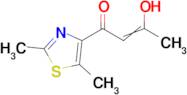
1-(2,5-dimethyl-1,3-thiazol-4-yl)-3-hydroxybut-2-en-1-one
Ref: 10-F683723
| 1g | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

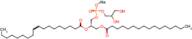
1-Palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt)
Ref: 10-F987899
| 25mg | Descontinuado | Solicitar informações | |
| 50mg | Descontinuado | Solicitar informações |

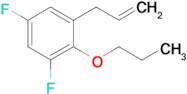
3-(3,5-Difluoro-2-n-propoxyphenyl)-1-propene
Ref: 10-F394884
| 1g | Descontinuado | Solicitar informações | |
| 5g | Descontinuado | Solicitar informações | |
| 25g | Descontinuado | Solicitar informações |

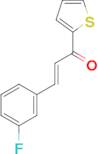
(2E)-3-(3-fluorophenyl)-1-(thiophen-2-yl)prop-2-en-1-one
Ref: 10-F524435
| 2g | Descontinuado | Solicitar informações | |
| 10g | Descontinuado | Solicitar informações |

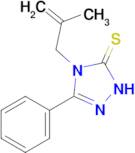
4-(2-methylprop-2-en-1-yl)-3-phenyl-4,5-dihydro-1H-1,2,4-triazole-5-thione
Ref: 10-F644790
| 1g | Descontinuado | Solicitar informações | |
| 50mg | Descontinuado | Solicitar informações | |
| 100mg | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

Ref: 10-F300461
| 1g | Descontinuado | Solicitar informações | |
| 5g | Descontinuado | Solicitar informações |

Ref: 10-F658248
| 1g | Descontinuado | Solicitar informações | |
| 50mg | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

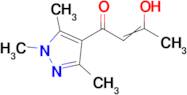
3-hydroxy-1-(1,3,5-trimethyl-1H-pyrazol-4-yl)but-2-en-1-one
Ref: 10-F680965
| 1g | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |

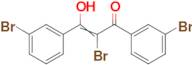
2-bromo-1,3-bis(3-bromophenyl)-3-hydroxyprop-2-en-1-one
Ref: 10-F678861
| 1g | Descontinuado | Solicitar informações | |
| 100mg | Descontinuado | Solicitar informações | |
| 250mg | Descontinuado | Solicitar informações | |
| 500mg | Descontinuado | Solicitar informações |



![ethyl 4-[(E)-2-(dimethylamino)vinyl]-8-(4-fluorophenyl)pyrazolo[5,1-c][1,2,4]triazine-3-carboxylate](https://static.cymitquimica.com/products/10/thumb/F371590.jpg)