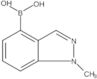
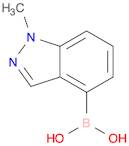
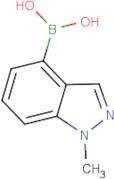
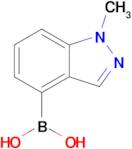
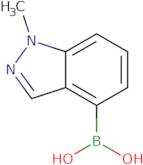
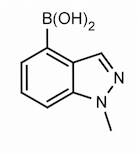
Boronic acid, B-(1-methyl-1H-indazol-4-yl)-
CAS:1001907-60-3
Formula:
C8H9BN2O2
Purity:
98%
Color and Shape:
Solid
Molecular weight:
175.9803
Ref: AN-AG00016I
| 1g | 68.00 € | |
| 5g | 193.00 € | |
| 100mg | 48.00 € | |
| 250mg | 50.00 € |
Estimated delivery in United States, on Monday 6 May 2024

Ref: FT-M11226
| 1g | To inquire | |
| 5g | To inquire |
Estimated delivery in United States, on Thursday 9 May 2024

1-Methyl-1H-indazole-4-boronic acid
CAS:1001907-60-3
Formula:
C8H9BN2O2
Purity:
≥99.0% (nmr) (Typical Value in Batch COA)
Color and Shape:
white to off white solid
Molecular weight:
175.98g/mol
Ref: 54-OR60109
| 1g | 99.00 € | |
| 5g | 300.00 € | |
| 250mg | 67.00 € |
Estimated delivery in United States, on Monday 13 May 2024

(1-Methyl-1H-indazol-4-yl)boronic acid
CAS:1001907-60-3
Purity:
97.0%
Color and Shape:
Solid, White to yellow solid
Molecular weight:
175.97999572753906
Ref: 10-F237003
| 1g | 64.00 € | |
| 5g | 190.00 € | |
| 10g | 333.00 € | |
| 250mg | To inquire |
Estimated delivery in United States, on Thursday 16 May 2024

1-Methylindazole-4-boronic acid
CAS:1001907-60-3
Please enquire for more information about 1-Methylindazole-4-boronic acid including the price, delivery time and more …
Formula:
C8H9BN2O2
Purity:
Min. 95%
Molecular weight:
175.98 g/mol
Ref: 3D-FM53520
| 1g | To inquire | |
| 50mg | To inquire | |
| 100mg | To inquire | |
| 250mg | To inquire | |
| 500mg | To inquire |
Estimated delivery in United States, on Monday 17 Jun 2024

1-Methyl-1H-indazole-4-boronic acid
CAS:1001907-60-3
Formula:
C8H9BN2O2
Purity:
98%
Molecular weight:
175.98
Ref: FT-FSIM11226
| 1g | Discontinued | |
| 5g | Discontinued |