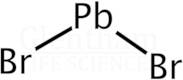
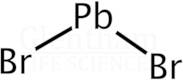
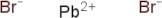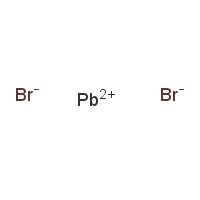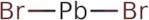CAS: 10031-22-8 - Lead(II) bromide
Formula:H2Br2Pb
InChI:InChI=1S/2BrH.Pb/h2*1H;/q;;+2/p-2
InChI key:InChIKey=ZASWJUOMEGBQCQ-UHFFFAOYSA-L
SMILES:Br[Pb]Br
- Synonyms:
- Bleidibromid
- Dibromo-Lambda~2~-Plumbane
- Dibromure de plomb
- Dibromuro De Plomo
- Lead Dibromide
- Lead bromide
- Lead bromide (PbBr2)
- Lead bromide (PbBr<sub>2</sub>)
- Leadbromide
- Leadbromidewhitepowder
- See more synonyms
- Plumbous bromide
- Tetrabromoplumbane

Lead(II) Bromide (Low water content) [for Perovskite precursor]
CAS:10031-22-8
Formula:
PbBr2
Purity:
>99.0%(T)
Color and Shape:
White to Almost white powder to crystal
Molecular weight:
367.01
Ref: 3B-L0346
| 1g | 59.00 € | |
| 5g | 175.00 € | |
| 25g | 624.00 € |
Estimated delivery in United States, on Thursday 2 May 2024

Lead(II) bromide, Puratronic™, 99.998% (metals basis)
CAS:10031-22-8
It is used in the field of antirust, pigment and photograph. The molten lead(II) bromide …
Formula:
Br2Pb
Purity:
99.998%
Color and Shape:
White to light brown, Powder
Molecular weight:
367.00
Ref: 02-010720
| 5g | 81.00 € | |
| 25g | 257.00 € |
Estimated delivery in United States, on Thursday 2 May 2024

Lead(II) bromide, 98+%
CAS:10031-22-8
It finds its uses as a catalyst to make polyesters, a filler for polypropylene (flame-resistant), …
Formula:
Br2Pb
Purity:
98+%
Color and Shape:
White, Crystals or powder or crystalline powder
Molecular weight:
367.00
Ref: 02-A19406
| 100g | 36.00 € | |
| 500g | 97.00 € |
Estimated delivery in United States, on Thursday 2 May 2024

Lead(II) bromide, ultra dry, 99.999% (metals basis)
CAS:10031-22-8
Lead(II) bromide is used in the field of antirust, pigment and photograph. The molten lead(II) …
Formula:
Br2Pb
Purity:
99.999%
Color and Shape:
White to pale grey, Beads
Molecular weight:
367.00
Ref: 02-035703
| 1g | 125.00 € | |
| 5g | 196.00 € | |
| 25g | 848.00 € |
Estimated delivery in United States, on Thursday 2 May 2024

Lead bromide (PbBr2)
CAS:10031-22-8
Formula:
Br2Pb
Purity:
99%
Color and Shape:
Solid
Molecular weight:
367.0080
Ref: AN-AG0001KN
| 1g | To inquire | |
| 5g | To inquire | |
| 25g | To inquire | |
| 100g | To inquire | |
| 500g | To inquire |
Estimated delivery in United States, on Wednesday 8 May 2024

Ref: 7W-GX7636
| Undefined size | To inquire |
Estimated delivery in United States, on Thursday 9 May 2024

Lead(II) bromide, 99.9%
CAS:10031-22-8
Formula:
PbBr2
Purity:
≥ 99.9%
Color and Shape:
White to off-white powder
Molecular weight:
367.01
Ref: 7W-GX2716
| Undefined size | To inquire |
Estimated delivery in United States, on Thursday 9 May 2024

Lead(II) bromide, 98+%
CAS:10031-22-8
Formula:
Br2Pb
Purity:
98+%
Color and Shape:
white pwdr.
Molecular weight:
367.01
Ref: 08-93-8203
| 50g | 122.00 € | |
| 250g | 439.00 € |
Estimated delivery in United States, on Thursday 9 May 2024

Lead(II) Bromide [for Perovskite precursor]
CAS:10031-22-8
Formula:
PbBr2
Purity:
>98.0%(T)
Color and Shape:
White to Almost white powder to crystaline
Molecular weight:
367.01
Ref: 3B-L0288
| 1g | 31.00 € | |
| 5g | 81.00 € | |
| 25g | 281.00 € |
Estimated delivery in United States, on Monday 13 May 2024

Lead(II) bromide
CAS:10031-22-8
Formula:
·Pb·2Br
Purity:
99.999%
Color and Shape:
white solid
Molecular weight:
367.01g/mol
Ref: 54-IN2209
| 10g | 293.00 € | |
| 25g | 477.00 € |
Estimated delivery in United States, on Wednesday 15 May 2024

Lead dibromide
CAS:10031-22-8
Lead dibromide is a non-radioactive catalyst that can be used in the organic synthesis of …
Formula:
PbBr2
Purity:
Min. 95%
Molecular weight:
367.01 g/mol
Ref: 3D-FL59683
| 25g | 157.00 € | |
| 50g | 271.00 € | |
| 100g | 387.00 € | |
| 250g | 592.00 € | |
| 500g | 939.00 € |
Estimated delivery in United States, on Thursday 20 Jun 2024










![Lead(II) Bromide (Low water content) [for Perovskite precursor]](https://static.cymitquimica.com/products/3B/thumb/L0346.jpg)
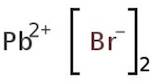
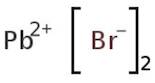
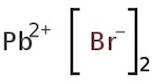

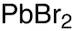
![Lead(II) Bromide [for Perovskite precursor]](https://static.cymitquimica.com/products/3B/thumb/L0288.jpg)