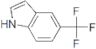
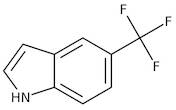
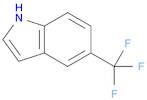
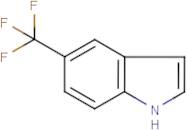
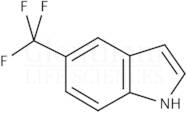
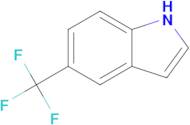
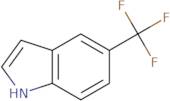
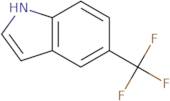
CAS 100846-24-0: 5-(Trifluoromethyl)indole
Description:5-(Trifluoromethyl)indole is an organic compound characterized by the presence of an indole ring substituted with a trifluoromethyl group at the 5-position. The indole structure consists of a fused benzene and pyrrole ring, contributing to its aromatic properties and potential biological activity. The trifluoromethyl group, which is a highly electronegative substituent, significantly influences the compound's chemical reactivity and lipophilicity, often enhancing its pharmacological properties. This compound is typically a solid at room temperature and is known for its stability under various conditions, although it may undergo reactions typical of indole derivatives, such as electrophilic substitution. Its unique structure makes it of interest in medicinal chemistry, particularly in the development of pharmaceuticals and agrochemicals. Additionally, the presence of fluorine atoms can improve metabolic stability and bioavailability, making it a valuable scaffold in drug design. Safety and handling precautions should be observed due to the potential toxicity associated with fluorinated compounds.
Formula:C8H7BrFNO
InChI:InChI=1/C8H7BrFNO/c1-5(12)11-8-3-2-6(10)4-7(8)9/h2-4H,1H3,(H,11,12)
- Synonyms:
- 1H-Indole, 5-(Triflu Oromethyl)- (9Ci)
- 5-Trifluoromethyl Indole ,98%
- N-(2-bromo-4-fluorophenyl)acetamide

5-(Trifluoromethyl)indole, 98%
Ref: 02-H28782
| 100mg | 106.00 € | ||
| 500mg | To inquire |

1H-Indole, 5-(trifluoromethyl)-
Ref: IN-DA0002ZS
| 1g | 62.00 € | ||
| 5g | 163.00 € | ||
| 10g | 190.00 € | ||
| 25g | 563.00 € | ||
| 50g | To inquire | ||
| 100g | To inquire | ||
| 50mg | 27.00 € | ||
| 100mg | 37.00 € | ||
| 250mg | 35.00 € |

5-(Trifluoromethyl)-1H-indole
Ref: 54-PC3045
| 1g | 53.00 € | ||
| 5g | 193.00 € | ||
| 25g | 696.00 € | ||
| 100g | 2,396.00 € | ||
| 250mg | 32.00 € |

5-(Trifluoromethyl)indole
Ref: 10-F216291
| 1g | 47.00 € | ||
| 5g | 170.00 € | ||
| 10g | 251.00 € | ||
| 25g | 563.00 € | ||
| 250mg | 21.00 € |

5-(Trifluoromethyl)indole
Ref: 3D-FT37897
| 5g | 256.00 € | ||
| 10g | 440.00 € | ||
| 25g | 753.00 € | ||
| 50g | 1,212.00 € | ||
| 100g | 1,968.00 € |

5-(Trifluoromethyl)indole
Ref: 3D-J-000232
| 1g | Discontinued | Request information | |
| 5g | Discontinued | Request information | |
| 10g | Discontinued | Request information | |
| 2500mg | Discontinued | Request information | |
| -Unit-g | Discontinued | Request information | |
| -Unit-gg | Discontinued | Request information |