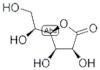
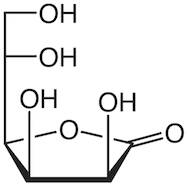
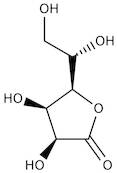
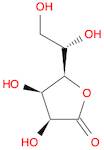
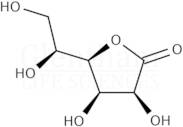
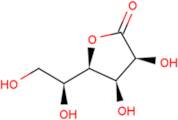
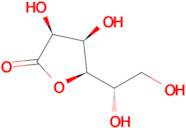
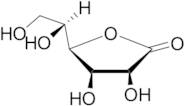
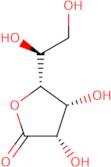
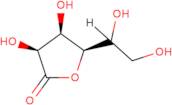
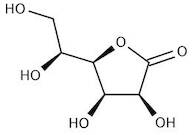
CAS: 1128-23-0 - L(+)-Gulonic acid gamma-lactone
Formula:C6H10O6
InChI:InChI=1S/C6H10O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2-5,7-10H,1H2/t2-,3+,4-,5+/m0/s1
InChI key:InChIKey=SXZYCXMUPBBULW-SKNVOMKLSA-N
SMILES:O=C1OC(C(O)CO)C(O)C1O
- Synonyms:
- (3R,4S,5S)-5-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxydihydrofuran-2(3H)-one (non-preferred name)
- (3S,4R,5R)-5-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxydihydrofuran-2(3H)-one (non-preferred name)
- <span class="text-smallcaps">L</span>-(+)-Gulonic acid γ-lactone
- <span class="text-smallcaps">L</span>-Gulono-1,4-lactone
- <span class="text-smallcaps">L</span>-Gulono-γ-lactone
- <span class="text-smallcaps">L</span>-Gulonolactone
- Dihydroascorbic acid
- Gulonic acid, γ-lactone, <span class="text-smallcaps">L</span>-
- L(+)-Gulonic acid-γ-lactone
- L(+)-Gulono-1,4-Lactone
- See more synonyms
- L-Gulonic Gamma-Lactone
- L-Gulonic-G-Lactone
- L-Gulono-Gamma-Lactone
- L-Gulonolactone
- Reduced ascorbate
- Reduced ascorbic acid
- γ-Gulonolactone

L-(+)-Gulonic Acid γ-Lactone
CAS:1128-23-0
Formula:
C6H10O6
Purity:
>98.0%(GC)
Color and Shape:
White to Almost white powder to crystal
Molecular weight:
178.14
Ref: 3B-G0235
| 5g | 49.00 € | |
| 25g | 212.00 € |
Estimated delivery in United States, on Thursday 25 Apr 2024

L-Gulonic acid-1,4-lactone, 95%
CAS:1128-23-0
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. …
Formula:
C6H10O6
Purity:
95%
Color and Shape:
Powder
Molecular weight:
178.14
Ref: 02-J66934
| 5g | 47.00 € | |
| 25g | 159.00 € |
Estimated delivery in United States, on Friday 26 Apr 2024

L(+)-Gulonic acid gamma-lactone
CAS:1128-23-0
Formula:
C6H10O6
Purity:
98%
Color and Shape:
Solid
Molecular weight:
178.1400
- Glycoscience
- Vitamins
- Monosaccharides
- Guloses
- See more categories
- Biochemicals and Reagents
Ref: AN-AG008R4F
| 1g | 21.00 € | |
| 5g | 27.00 € | |
| 25g | 67.00 € | |
| 100g | 123.00 € | |
| 500g | 463.00 € |
Estimated delivery in United States, on Thursday 2 May 2024

L-Gulono-1,4-lactone
CAS:1128-23-0
Formula:
C6H10O6
Purity:
≥ 90.0%
Color and Shape:
White to off-white crystalline powder
Molecular weight:
178.14
Ref: 7W-GC8500
| Undefined size | To inquire |
Estimated delivery in United States, on Friday 3 May 2024

L-Gulono-1,4-lactone
CAS:1128-23-0
L-Gulono-1,4-lactone is a substrate of L-gulono-1,4-lactone oxidoreductase, which catalyzes the last step of the biosynthesis …
Formula:
C6H10O6
Purity:
98%
Color and Shape:
Less Crystals Colorless Crystals
Molecular weight:
178.14
Ref: TM-T5578
| 1g | 54.00 € | |
| 500mg | 49.00 € | |
| 1ml*10 (DMSO) | 54.00 € |
Estimated delivery in United States, on Thursday 9 May 2024

(3S,4R,5R)-5-((S)-1,2-Dihydroxyethyl)-3,4-dihydroxydihydrofuran-2(3H)-one
CAS:1128-23-0
Purity:
95.0%
Color and Shape:
Solid
Molecular weight:
178.13999938964844
- Cyclic Compounds
- Esters
- 5-membered Heterocycles
- Furan
- See more categories
- Tetrahydrofuran
Ref: 10-F233102
| 5g | To inquire | |
| 25g | To inquire | |
| 100g | To inquire | |
| 500g | To inquire |
Estimated delivery in United States, on Tuesday 14 May 2024

L-Gulono-1,4-lactone
Controlled ProductCAS:1128-23-0
Applications L-Gulono-1,4-lactone is a precursor in the biosynthesis of ascorbic acid, one of the many …
Formula:
C6H10O6
Color and Shape:
White to Off-White Solid
Molecular weight:
178.14
Ref: TR-G855000
| 5g | 117.00 € | |
| 25g | 242.00 € | |
| 100g | 730.00 € |
Estimated delivery in United States, on Tuesday 14 May 2024

L-Gulonic acid-1,4-lactone
CAS:1128-23-0
L-Gulonic acid-1,4-lactone is an ascorbic acid derivative that inhibits the production of matrix metalloproteinases (MMPs) …
Formula:
C6H10O6
Purity:
Min. 95 Area-%
Color and Shape:
White Powder
Molecular weight:
178.14 g/mol
Ref: 3D-MG06707
| 1kg | 248.00 € | |
| 2kg | 381.00 € | |
| 100g | 107.00 € | |
| 250g | 140.00 € | |
| 500g | 188.00 € |
Estimated delivery in United States, on Monday 13 May 2024

L-Gulono-gamma-lactone
CAS:1128-23-0
L-Gulono-gamma-lactone is a natural vitamin C metabolite that is synthesized from L-ascorbic acid in the …
Formula:
C6H10O6
Molecular weight:
178.14 g/mol
Ref: 3D-G-8500
| 1kg | To inquire | |
| 100g | To inquire | |
| 250g | To inquire | |
| 500g | To inquire | |
| 2500g | To inquire |
Estimated delivery in United States, on Monday 13 May 2024

L-Gulono-1,4-Lactone extrapure, 98%
CAS:1128-23-0
Formula:
C6H10O6
Purity:
min. 98%
Color and Shape:
White to off - white, Crystalline powder
Molecular weight:
178.14
Ref: SR-97514
| 10g | 74.00 € | |
| 25g | 132.00 € |
Estimated delivery in United States, on Thursday 30 May 2024

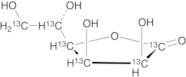
L-Gulono-1,4-lactone-13C6
Controlled ProductCAS:1128-23-0
Applications Isotope labelled L-Gulono-1,4-lactone, a precursor in the biosynthesis of ascorbic acid, one of the …
Formula:
C6H10O6
Color and Shape:
White to Off-White Solid
Molecular weight:
184.1
Ref: TR-G855002
| 5mg | 227.00 € | |
| 10mg | 383.00 € | |
| 50mg | 1,601.00 € |
Estimated delivery in United States, on Wednesday 12 Jun 2024