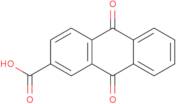
ANTHRAQUINONE-2-CARBOXYLIC ACID
CAS: 117-78-2
Ref. 3D-FA43912
| 1g | Discontinued | ||
| 250mg | Discontinued | ||
| 500mg | Discontinued |
Product Information
- 2-Anthracenecarboxylic acid, 9,10-dihydro-9,10-dioxo-
- 2-Anthraquinone Carboxylic Acid
- 2-Anthraquinonecarboxylic acid
- 2-Anthroic acid, 9,10-dihydro-9,10-dioxo-
- 2-Carboxy-9,10-anthraquinone
- 2-Carboxyanthraquinone
- 9,10-Anthraquinone-2-carboxylic acid
- 9,10-Dihydro-9,10-Dioxo-2-Anthra cenecarboxylic Acid
- 9,10-Dihydro-9,10-Dioxo-2-Anthroic Acid
- 9,10-Dihydro-9,10-dioxo-2-anthracenecarboxylic acid
- See more synonyms
- 9,10-Dioxo-9,10-Dihydroanthracene-2-Carboxylic Acid
- 9,10-Dioxo-9,10-dihydro-2-anthracenecarboxylic acid
- 9,10-Dioxoanthracene-2-carboxylic acid
- Beta-9,10-Dioxoanthracene-2-Carboxylic Acid
- NSC 5001
- β-Anthraquinonecarboxylic acid
Anthraquinone-2-carboxylate is a metabolite of the anti-tuberculosis drug ethambutol. It is an aromatic compound that has been shown to have cytotoxic effects on human tumour cells in urine samples and wastewater treatment. Anthraquinone-2-carboxylate is stable in complexes with metal ions such as ferrocene carboxylic acid and nitrite reductase, and it reacts with hydrogen to form a molecule with intramolecular hydrogen bonds. This reaction mechanism also leads to the formation of carboxylates, which are important components of many organic molecules such as amino acids, hormones, enzymes, and vitamins. Anthraquinone-2-carboxylate can be used to produce stable complexes with metal ions that may be used in reactions involving nitrite reductase or ferrocenecarboxylic acid. The stability of these complexes make them useful for electron transfer reactions where they act